
A device to check battery voltage.
A battery's capacity is the amount of electric charge it can store. The more electrolyte and electrode material there is in the cell the greater the capacity of the cell. A small cell has less capacity than a larger cell with the same chemistry, and they develop the same open-circuit voltage.[43]
Because of the chemical reactions within the cells, the capacity of a battery depends on the discharge conditions such as the magnitude of the current (which may vary with time), the allowable terminal voltage of the battery, temperature, and other factors.[43] The available capacity of a battery depends upon the rate at which it is discharged.[44] If a battery is discharged at a relatively high rate, the available capacity will be lower than expected.
The capacity printed on a battery is usually the product of 20 hours multiplied by the constant current that a new battery can supply for 20 hours at 68 F° (20 C°), down to a specified terminal voltage per cell. A battery rated at 100 A·h will deliver 5 A over a 20-hour period at room temperature. However, if discharged at 50 A, it will have a lower capacity.[45]
The relationship between current, discharge time, and capacity for a lead acid battery is approximated (over a certain range of current values) by Peukert's law:
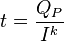
where
 is the capacity when discharged at a rate of 1 amp.
is the capacity when discharged at a rate of 1 amp. 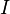 is the current drawn from battery (A).
is the current drawn from battery (A).  is the amount of time (in hours) that a battery can sustain.
is the amount of time (in hours) that a battery can sustain. 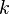 is a constant around 1.3.
is a constant around 1.3.
For low values of I internal self-discharge must be included.
Internal energy losses and limited rate of diffusion of ions through the electrolyte cause the efficiency of a real battery to vary at different discharge rates. When discharging at low rate, the battery's energy is delivered more efficiently than at higher discharge rates,[45] but if the rate is very low, it will partly self-discharge during the long time of operation, again lowering its efficiency.
Installing batteries with different A·h ratings will not affect the operation of a device (except for the time it will work for) rated for a specific voltage unless the load limits of the battery are exceeded. High-drain loads such as digital cameras can result in delivery of less total energy, as happens with alkaline batteries.[31] For example, a battery rated at 2000 mA·h for a 10- or 20-hour discharge would not sustain a current of 1 A for a full two hours as its stated capacity implies.